Research
Signaling
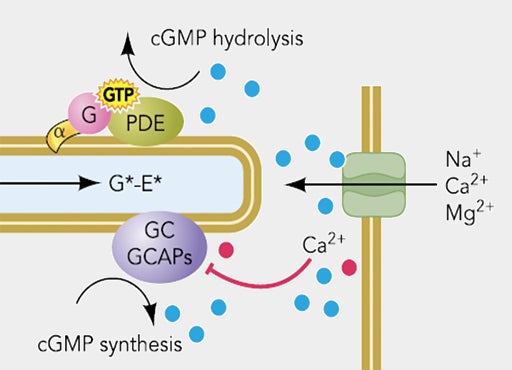
Environment matters. Photoreceptors transduce photons into electrical signals, which also requires them to engage in bi-directional signaling with other cell types, including retinal pigmented epithelium and Müller glial cells. We are studying the biophysical mechanisms of intracellular and intercellular signaling in vivo.
Degeneration
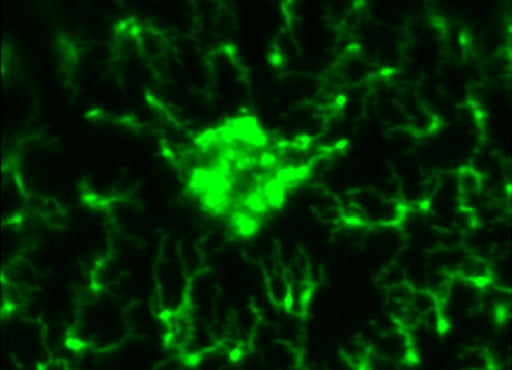
As photoreceptors degenerate, they trigger activation of resident microglia and infiltration of monocytes from the bloodstream. We are studying this inflammatory response across spatial and temporal scales.